

You can:
| Name | CHEMBL1084552 |
|---|---|
| Molecular formula | C26H20ClF3N2O4 |
| IUPAC name | 4-[(1S)-1-[[1-[[3-chloro-5-(trifluoromethoxy)phenyl]methyl]indole-7-carbonyl]amino]ethyl]benzoic acid |
| Molecular weight | 516.901 |
| Hydrogen bond acceptor | 7 |
| Hydrogen bond donor | 2 |
| XlogP | 6.1 |
| Synonyms | BDBM50319840 (S)-4-(1-(1-(3-chloro-5-(trifluoromethoxy)benzyl)-1H-indole-7-carboxamido)ethyl)benzoic acid |
| Inchi Key | HQCZRLVYPNLNQN-HNNXBMFYSA-N |
| Inchi ID | InChI=1S/C26H20ClF3N2O4/c1-15(17-5-7-19(8-6-17)25(34)35)31-24(33)22-4-2-3-18-9-10-32(23(18)22)14-16-11-20(27)13-21(12-16)36-26(28,29)30/h2-13,15H,14H2,1H3,(H,31,33)(H,34,35)/t15-/m0/s1 |
| PubChem CID | 46890658 |
| ChEMBL | CHEMBL1084552 |
| IUPHAR | N/A |
| BindingDB | 50319840 |
| DrugBank | N/A |
Structure | 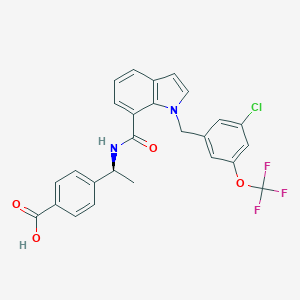 |
| Lipinski's druglikeness | This ligand is heavier than 500 daltons. This ligand has a partition coefficient log P greater than 5. |
You can:
| GLASS ID | Name | UniProt | Gene | Species | Length |
|---|---|---|---|---|---|
| 121274 | Prostacyclin receptor | P43119 | PTGIR | Homo sapiens (Human) | 386 |
| 121267 | Prostaglandin D2 receptor | Q13258 | PTGDR | Homo sapiens (Human) | 359 |
| 121269 | Prostaglandin D2 receptor 2 | Q9Y5Y4 | PTGDR2 | Homo sapiens (Human) | 395 |
| 121272 | Prostaglandin E2 receptor EP1 subtype | P34995 | PTGER1 | Homo sapiens (Human) | 402 |
| 121273 | Prostaglandin E2 receptor EP2 subtype | P43116 | PTGER2 | Homo sapiens (Human) | 358 |
| 121271 | Prostaglandin E2 receptor EP3 subtype | P43115 | PTGER3 | Homo sapiens (Human) | 390 |
| 121270 | Prostaglandin E2 receptor EP4 subtype | P35408 | PTGER4 | Homo sapiens (Human) | 488 |
| 121268 | Prostaglandin F2-alpha receptor | P43088 | PTGFR | Homo sapiens (Human) | 359 |
| 121275 | Thromboxane A2 receptor | P21731 | TBXA2R | Homo sapiens (Human) | 343 |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417