

You can:
| Name | Alpha-1A adrenergic receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADRA1A |
| Synonym | alpha1a ADRA1C ADRA1L1 adrenergic alpha 1c receptor adrenergic receptor alpha 1c [ Show all ] |
| Disease | Urinary incontinence Benign prostatic hyperplasia Cognitive disorders Female sexual dysfunction Glaucoma [ Show all ] |
| Length | 466 |
| Amino acid sequence | MVFLSGNASDSSNCTQPPAPVNISKAILLGVILGGLILFGVLGNILVILSVACHRHLHSVTHYYIVNLAVADLLLTSTVLPFSAIFEVLGYWAFGRVFCNIWAAVDVLCCTASIMGLCIISIDRYIGVSYPLRYPTIVTQRRGLMALLCVWALSLVISIGPLFGWRQPAPEDETICQINEEPGYVLFSALGSFYLPLAIILVMYCRVYVVAKRESRGLKSGLKTDKSDSEQVTLRIHRKNAPAGGSGMASAKTKTHFSVRLLKFSREKKAAKTLGIVVGCFVLCWLPFFLVMPIGSFFPDFKPSETVFKIVFWLGYLNSCINPIIYPCSSQEFKKAFQNVLRIQCLCRKQSSKHALGYTLHPPSQAVEGQHKDMVRIPVGSRETFYRISKTDGVCEWKFFSSMPRGSARITVSKDQSSCTTARVRSKSFLQVCCCVGPSTPSLDKNHQVPTIKVHTISLSENGEEV |
| UniProt | P35348 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P35348 |
| 3D structure model | This predicted structure model is from GPCR-EXP P35348. |
| BioLiP | N/A |
| Therapeutic Target Database | T92609 |
| ChEMBL | CHEMBL229 |
| IUPHAR | 22 |
| DrugBank | BE0000501 |
| Name | tetrahydropalmatine |
|---|---|
| Molecular formula | C21H25NO4 |
| IUPAC name | (13aS)-2,3,9,10-tetramethoxy-6,8,13,13a-tetrahydro-5H-isoquinolino[2,1-b]isoquinoline |
| Molecular weight | 355.434 |
| Hydrogen bond acceptor | 5 |
| Hydrogen bond donor | 0 |
| XlogP | 3.2 |
| Synonyms | 6H-Dibenzo[a,g]quinolizine,5,8,13,13a-tetrahydro-2,3,9,10-tetramethoxy-, (13aS)- AKOS000277105 Caseanine Hyndarin LS-61256 [ Show all ] |
| Inchi Key | AEQDJSLRWYMAQI-KRWDZBQOSA-N |
| Inchi ID | InChI=1S/C21H25NO4/c1-23-18-6-5-13-9-17-15-11-20(25-3)19(24-2)10-14(15)7-8-22(17)12-16(13)21(18)26-4/h5-6,10-11,17H,7-9,12H2,1-4H3/t17-/m0/s1 |
| PubChem CID | 72301 |
| ChEMBL | CHEMBL487182 |
| IUPHAR | N/A |
| BindingDB | 50424077 |
| DrugBank | DB12093 |
Structure | 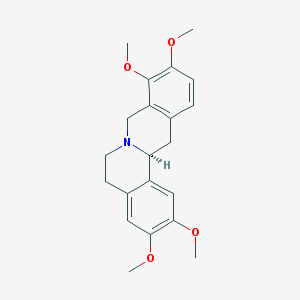 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Inhibition | 41.2 % | PMID27709945 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417