

You can:
| Name | Neuropeptide S receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | NPSR1 |
| Synonym | G-protein coupled receptor for asthma susceptibility vasopressin receptor-related receptor 1 G protein-coupled receptor 154 PGR14 NPS receptor [ Show all ] |
| Disease | Neurological disease |
| Length | 371 |
| Amino acid sequence | MPANFTEGSFDSSGTGQTLDSSPVACTETVTFTEVVEGKEWGSFYYSFKTEQLITLWVLFVFTIVGNSVVLFSTWRRKKKSRMTFFVTQLAITDSFTGLVNILTDINWRFTGDFTAPDLVCRVVRYLQVVLLYASTYVLVSLSIDRYHAIVYPMKFLQGEKQARVLIVIAWSLSFLFSIPTLIIFGKRTLSNGEVQCWALWPDDSYWTPYMTIVAFLVYFIPLTIISIMYGIVIRTIWIKSKTYETVISNCSDGKLCSSYNRGLISKAKIKAIKYSIIIILAFICCWSPYFLFDILDNFNLLPDTQERFYASVIIQNLPALNSAINPLIYCVFSSSISFPCREQRSQDSRMTFRERTERHEMQILSKPEFI |
| UniProt | Q6W5P4 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | Q6W5P4 |
| 3D structure model | This predicted structure model is from GPCR-EXP Q6W5P4. |
| BioLiP | N/A |
| Therapeutic Target Database | T20958 |
| ChEMBL | CHEMBL5162 |
| IUPHAR | 302 |
| DrugBank | BE0000948 |
| Name | PK 11195 |
|---|---|
| Molecular formula | C21H21ClN2O |
| IUPAC name | N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide |
| Molecular weight | 352.862 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 0 |
| XlogP | 5.5 |
| Synonyms | BRD-A41451487-001-01-3 DSSTox_RID_79625 HMS3260H17 MFCD00069334 NCGC00015205-04 [ Show all ] |
| Inchi Key | RAVIZVQZGXBOQO-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 |
| PubChem CID | 1345 |
| ChEMBL | CHEMBL481537 |
| IUPHAR | N/A |
| BindingDB | 22032 |
| DrugBank | N/A |
Structure | 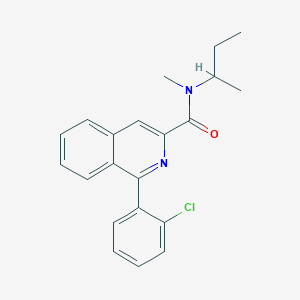 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Potency | 199.5 nM | PubChem BioAssay data set | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417