

You can:
| Name | Cannabinoid receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CNR1 |
| Synonym | CB1 Central cannabinoid receptor SKR6R THC receptor CB1R [ Show all ] |
| Disease | Obesity; Diabetes Chemotherapy-induced nausea Diabetes; Obesity Drug abuse Hypertension; Diabetes; Obesity [ Show all ] |
| Length | 472 |
| Amino acid sequence | MKSILDGLADTTFRTITTDLLYVGSNDIQYEDIKGDMASKLGYFPQKFPLTSFRGSPFQEKMTAGDNPQLVPADQVNITEFYNKSLSSFKENEENIQCGENFMDIECFMVLNPSQQLAIAVLSLTLGTFTVLENLLVLCVILHSRSLRCRPSYHFIGSLAVADLLGSVIFVYSFIDFHVFHRKDSRNVFLFKLGGVTASFTASVGSLFLTAIDRYISIHRPLAYKRIVTRPKAVVAFCLMWTIAIVIAVLPLLGWNCEKLQSVCSDIFPHIDETYLMFWIGVTSVLLLFIVYAYMYILWKAHSHAVRMIQRGTQKSIIIHTSEDGKVQVTRPDQARMDIRLAKTLVLILVVLIICWGPLLAIMVYDVFGKMNKLIKTVFAFCSMLCLLNSTVNPIIYALRSKDLRHAFRSMFPSCEGTAQPLDNSMGDSDCLHKHANNAASVHRAAESCIKSTVKIAKVTMSVSTDTSAEAL |
| UniProt | P21554 |
| Protein Data Bank | 5tjv, 5u09, 5xr8, 5xra, 6n4b, 5tgz |
| GPCR-HGmod model | P21554 |
| 3D structure model | This structure is from PDB ID 5tjv. |
| BioLiP | BL0384680, BL0364157, BL0384679, BL0384681, BL0384682, BL0384683, BL0384684, BL0440253, BL0440254,BL0440255, BL0363267, BL0361447, BL0361446 |
| Therapeutic Target Database | T76685 |
| ChEMBL | CHEMBL218 |
| IUPHAR | 56 |
| DrugBank | BE0000061 |
| Name | 362519-49-1 |
|---|---|
| Molecular formula | C23H20Cl2N4O2S |
| IUPAC name | 5-(4-chlorophenyl)-N-(4-chlorophenyl)sulfonyl-N'-methyl-4-phenyl-3,4-dihydropyrazole-2-carboximidamide |
| Molecular weight | 487.399 |
| Hydrogen bond acceptor | 4 |
| Hydrogen bond donor | 1 |
| XlogP | 5.3 |
| Synonyms | ( inverted exclamation markA)-SLV-319 MolPort-035-765-770 (+/-)-SLV319 CTK8E8422 RT-015587 [ Show all ] |
| Inchi Key | AXJQVVLKUYCICH-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) |
| PubChem CID | 11179267 |
| ChEMBL | CHEMBL158784 |
| IUPHAR | N/A |
| BindingDB | 29094 |
| DrugBank | N/A |
Structure | 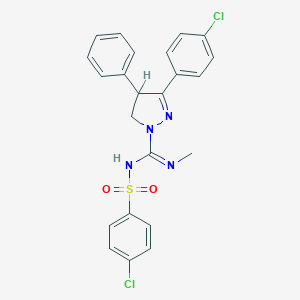 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| IC50 | 1.905 nM | PMID19406638 | ChEMBL |
| IC50 | 1.91 nM | PMID19406638 | BindingDB |
| IC50 | 22.0 nM | PMID22959249 | BindingDB,ChEMBL |
| IC50 | 139.0 nM | PMID22959249 | BindingDB,ChEMBL |
| Kd | 1.995 nM | PMID20047331, PMID20137935, PMID19699640, PMID14736243 | ChEMBL |
| Ki | 3.0 nM | PMID19338356 | BindingDB |
| Ki | 4.1 nM | PMID18083560 | PDSP |
| Ki | 4.1 nM | PMID18083560 | BindingDB |
| Ki | 7.8 nM | PMID19520572 | PDSP |
| Ki | 8.0 nM | PMID18335976 | PDSP,BindingDB |
| Ki | 25.0 nM | PMID20047331, PMID19699640, PMID14736243 | PDSP,BindingDB,ChEMBL |
| Ki | 25.2 nM | PMID20137935, PMID14736243 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417