

You can:
| Name | D(1A) dopamine receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | DRD1 |
| Synonym | D1 receptor D1A DADR Gpcr15 dopamine D1 receptor [ Show all ] |
| Disease | Unspecified Hypertension Pain Parkinson's disease Psychiatric disorder [ Show all ] |
| Length | 446 |
| Amino acid sequence | MRTLNTSAMDGTGLVVERDFSVRILTACFLSLLILSTLLGNTLVCAAVIRFRHLRSKVTNFFVISLAVSDLLVAVLVMPWKAVAEIAGFWPFGSFCNIWVAFDIMCSTASILNLCVISVDRYWAISSPFRYERKMTPKAAFILISVAWTLSVLISFIPVQLSWHKAKPTSPSDGNATSLAETIDNCDSSLSRTYAISSSVISFYIPVAIMIVTYTRIYRIAQKQIRRIAALERAAVHAKNCQTTTGNGKPVECSQPESSFKMSFKRETKVLKTLSVIMGVFVCCWLPFFILNCILPFCGSGETQPFCIDSNTFDVFVWFGWANSSLNPIIYAFNADFRKAFSTLLGCYRLCPATNNAIETVSINNNGAAMFSSHHEPRGSISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT |
| UniProt | P21728 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P21728 |
| 3D structure model | This predicted structure model is from GPCR-EXP P21728. |
| BioLiP | N/A |
| Therapeutic Target Database | T22118 |
| ChEMBL | CHEMBL2056 |
| IUPHAR | 214 |
| DrugBank | BE0000020 |
| Name | raloxifene |
|---|---|
| Molecular formula | C28H27NO4S |
| IUPAC name | [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone |
| Molecular weight | 473.587 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 2 |
| XlogP | 6.1 |
| Synonyms | LY-156758 NCGC00015889-02 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]-thiophene NCGC00092353-04 82640-04-8 (hydrochloride) [ Show all ] |
| Inchi Key | GZUITABIAKMVPG-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 |
| PubChem CID | 5035 |
| ChEMBL | CHEMBL81 |
| IUPHAR | 2820 |
| BindingDB | 19441 |
| DrugBank | N/A |
Structure | 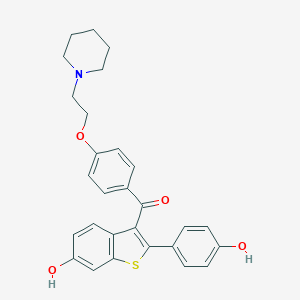 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | -7.2 % | PMID24088053 | ChEMBL |
| IC50 | 3864.0 nM | DrugMatrix in vitro pharmacology data | ChEMBL |
| Inhibition | 97.0 % | PMID24088053 | ChEMBL |
| Ki | 1932.0 nM | DrugMatrix in vitro pharmacology data | ChEMBL |
| Potency | 231.1 nM | PubChem BioAssay data set | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417