

You can:
| Name | Xanthine amine congener |
|---|---|
| Molecular formula | C21H28N6O4 |
| IUPAC name | N-(2-aminoethyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide |
| Molecular weight | 428.493 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 3 |
| XlogP | 2.7 |
| Synonyms | ZINC9210767 Acetamide, N-(2-aminoethyl)-2-(4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy)- DTXSID10242595 LP01279 N-(2-Aminoethyl)-2-[4-(2,6-dioxo-1,3-dipropyl-2,3,6,9-tetrahydro-1H-purin-8-yl)phenoxy]acetamide # [ Show all ] |
| Inchi Key | FIQGIOAELHTLHM-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) |
| PubChem CID | 5697 |
| ChEMBL | CHEMBL273094 |
| IUPHAR | 404, 432 |
| BindingDB | 50207816 |
| DrugBank | N/A |
Structure | 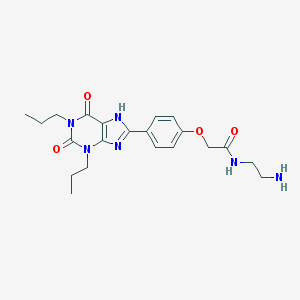 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Kd | 10.0 nM | PMID7775460 | IUPHAR |
| Kd | 18.46 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Kd | 19.95 nM | PMID21661720 | ChEMBL |
| Kd | 76.29 nM | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| Ki | 1.0 nM | PMID23200243, PMID9459566 | PDSP,BindingDB,ChEMBL |
| Ki | 1.0 - 3.98107 nM | PMID9459566, PMID9179373 | IUPHAR |
| Ki | 3.7 nM | PMID23200243 | ChEMBL |
| Ki | 5.8 nM | PMID23602401 | ChEMBL |
| Ki | 7.89 nM | PMID9258366 | ChEMBL |
| Ki | 7.9 nM | PMID9258366 | BindingDB |
| Ki | 15.6 nM | PMID9258366 | ChEMBL |
| Ki | 16.0 nM | PMID9258366 | BindingDB |
| Ki | 18.4 nM | PMID10737749 | BindingDB,ChEMBL |
| Ki | 24.0 nM | PMID2825043 | BindingDB |
| Ki | 25.12 nM | PMID21661720 | BindingDB,ChEMBL |
| Ki | 45.6 nM | PMID9258366 | ChEMBL |
| Ki | 46.0 nM | PMID9258366 | BindingDB |
| Ki | 63.0 nM | PMID10956189 | BindingDB,ChEMBL |
| koff | 0.1578 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 0.01558 nM^-1 min^-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 482900.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| kon | 915967.0 Ms-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.0165 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
| k_off | 0.03484 s-1 | DOI: http://dx.doi.org/10.6019/CHEMBL3885741 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417