

You can:
| Name | Type-2 angiotensin II receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | AGTR2 |
| Synonym | AT2 MRX88 Agtr2 angiotensin II receptor angiotensin II receptor, type 2 [ Show all ] |
| Disease | Postherpetic neuralgia Hypertension |
| Length | 363 |
| Amino acid sequence | MKGNSTLATTSKNITSGLHFGLVNISGNNESTLNCSQKPSDKHLDAIPILYYIIFVIGFLVNIVVVTLFCCQKGPKKVSSIYIFNLAVADLLLLATLPLWATYYSYRYDWLFGPVMCKVFGSFLTLNMFASIFFITCMSVDRYQSVIYPFLSQRRNPWQASYIVPLVWCMACLSSLPTFYFRDVRTIEYLGVNACIMAFPPEKYAQWSAGIALMKNILGFIIPLIFIATCYFGIRKHLLKTNSYGKNRITRDQVLKMAAAVVLAFIICWLPFHVLTFLDALAWMGVINSCEVIAVIDLALPFAILLGFTNSCVNPFLYCFVGNRFQQKLRSVFRVPITWLQGKRESMSCRKSSSLREMETFVS |
| UniProt | P50052 |
| Protein Data Bank | 5xjm, 5unh, 5ung, 5unf |
| GPCR-HGmod model | P50052 |
| 3D structure model | This structure is from PDB ID 5xjm. |
| BioLiP | BL0419199, BL0375199,BL0375200, BL0375198, BL0375196,BL0375197 |
| Therapeutic Target Database | T09909 |
| ChEMBL | CHEMBL4607 |
| IUPHAR | 35 |
| DrugBank | BE0003426 |
| Name | CHEMBL74476 |
|---|---|
| Molecular formula | C35H43N5O6S |
| IUPAC name | butyl N-[2-[4-[[2-butyl-6-[[methyl(propan-2-yl)carbamoyl]amino]-4-oxoquinazolin-3-yl]methyl]phenyl]phenyl]sulfonylcarbamate |
| Molecular weight | 661.818 |
| Hydrogen bond acceptor | 7 |
| Hydrogen bond donor | 2 |
| XlogP | 5.8 |
| Synonyms | L010012 2-Butyl-3-(2''-(butyloxycarbonylaminosulphonyl)-biphenyl-4-ylmethyl)-6-(N-isopropyl-N-methyl-aminocarbonylamino)-3H-quinazolin-4-one BDBM50049181 L-162393 4''-[2-Butyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-4H-quinazolin-3-ylmethyl]-biphenyl-2-sulfonic acid butyloxycarbonylamide(LP-162,393) |
| Inchi Key | AEJPLKVXLXBIJD-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C35H43N5O6S/c1-6-8-14-32-37-30-20-19-27(36-34(42)39(5)24(3)4)22-29(30)33(41)40(32)23-25-15-17-26(18-16-25)28-12-10-11-13-31(28)47(44,45)38-35(43)46-21-9-7-2/h10-13,15-20,22,24H,6-9,14,21,23H2,1-5H3,(H,36,42)(H,38,43) |
| PubChem CID | 9895984 |
| ChEMBL | CHEMBL74476 |
| IUPHAR | N/A |
| BindingDB | 50049181 |
| DrugBank | N/A |
Structure | 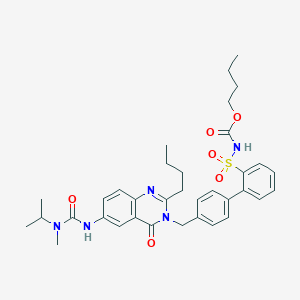 |
| Lipinski's druglikeness | This ligand is heavier than 500 daltons. This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| IC50 | 60.0 nM | , Bioorg. Med. Chem. Lett., (1994) 4:19:2337 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417