

You can:
| Name | 5-hydroxytryptamine receptor 1D |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HTR1D |
| Synonym | Serotonin 1D alpha receptor serotonin receptor 1D HTRL Htr1db 5-HT-1D [ Show all ] |
| Disease | Acute migraine Epilepsy Migraine headaches Mood disorder Migraine [ Show all ] |
| Length | 377 |
| Amino acid sequence | MSPLNQSAEGLPQEASNRSLNATETSEAWDPRTLQALKISLAVVLSVITLATVLSNAFVLTTILLTRKLHTPANYLIGSLATTDLLVSILVMPISIAYTITHTWNFGQILCDIWLSSDITCCTASILHLCVIALDRYWAITDALEYSKRRTAGHAATMIAIVWAISICISIPPLFWRQAKAQEEMSDCLVNTSQISYTIYSTCGAFYIPSVLLIILYGRIYRAARNRILNPPSLYGKRFTTAHLITGSAGSSLCSLNSSLHEGHSHSAGSPLFFNHVKIKLADSALERKRISAARERKATKILGIILGAFIICWLPFFVVSLVLPICRDSCWIHPALFDFFTWLGYLNSLINPIIYTVFNEEFRQAFQKIVPFRKAS |
| UniProt | P28221 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P28221 |
| 3D structure model | This predicted structure model is from GPCR-EXP P28221. |
| BioLiP | N/A |
| Therapeutic Target Database | T11072 |
| ChEMBL | CHEMBL1983 |
| IUPHAR | 3 |
| DrugBank | BE0000659 |
| Name | serotonin |
|---|---|
| Molecular formula | C10H12N2O |
| IUPAC name | 3-(2-aminoethyl)-1H-indol-5-ol |
| Molecular weight | 176.219 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 3 |
| XlogP | 0.2 |
| Synonyms | Lopac0_000607 AC1Q54C0 NCGC00015525-05 AX8011985 NCGC00142449-04 [ Show all ] |
| Inchi Key | QZAYGJVTTNCVMB-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 |
| PubChem CID | 5202 |
| ChEMBL | CHEMBL39 |
| IUPHAR | 5 |
| BindingDB | 10755 |
| DrugBank | DB08839 |
Structure | 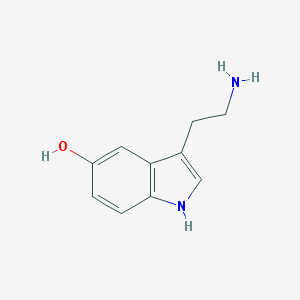 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| ED50 | 1.2 nM | PMID8071931 | ChEMBL |
| IA | 1.0 - | PMID15887956 | ChEMBL |
| IC50 | 3.0 nM | , PMID2374139, Bioorg. Med. Chem. Lett., (1995) 5:20:2391 | BindingDB,ChEMBL |
| Ki | 0.5 nM | PMID18507369 | BindingDB |
| Ki | 0.5 nM | PMID18507369 | PDSP,ChEMBL |
| Ki | 1.0 - 10.0 nM | PMID10431754, PMID1565658, PMID9208142, PMID9776361, PMID8957260, PMID8967979, PMID1652050 | IUPHAR |
| Ki | 1.2 nM | Peroutka et al., PMID1989 | PDSP |
| Ki | 1.7 nM | PMID8568822 | BindingDB,ChEMBL |
| Ki | 2.0 nM | PMID9986723 | BindingDB,ChEMBL |
| Ki | 2.08 nM | PMID7984267 | PDSP,BindingDB |
| Ki | 2.1 nM | PMID1652050 | PDSP,BindingDB |
| Ki | 3.0 nM | PMID2299641 | BindingDB,ChEMBL |
| Ki | 3.38 nM | PMID9776361 | PDSP,BindingDB |
| Ki | 3.4 nM | PMID9303569 | PDSP,BindingDB |
| Ki | 3.89 nM | PMID7984267 | PDSP,BindingDB |
| Ki | 3.98 nM | PMID9303567 | PDSP,BindingDB |
| Ki | 4.3 nM | PMID1565658 | PDSP,BindingDB |
| Ki | 4.8 nM | PMID7658447 | BindingDB,ChEMBL |
| Ki | 5.06 nM | PMID8960551, PMID9871775 | BindingDB,ChEMBL |
| Ki | 5.1 nM | PMID9871775 | BindingDB |
| Ki | 5.5 nM | PMID8071931 | BindingDB,ChEMBL |
| Ki | 6.46 nM | Waeber et al., PMID1988 | PDSP |
| Ki | 8.9 nM | PMID1652050 | PDSP,BindingDB |
| Ki | 8.91 nM | PMID7984267 | PDSP,BindingDB |
| pKD | 7.9 - | PMID8515429 | ChEMBL |
| Selectivity | 0.4 - | PMID8568822 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417