

You can:
| Name | Nociceptin receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | OPRL1 |
| Synonym | Orphanin FQ receptor OP4 NOPr NOP-r NOP receptor [ Show all ] |
| Disease | Inflammatory disease Major depressive disorder Central nervous system disease Heart failure Anxiety disorder [ Show all ] |
| Length | 370 |
| Amino acid sequence | MEPLFPAPFWEVIYGSHLQGNLSLLSPNHSLLPPHLLLNASHGAFLPLGLKVTIVGLYLAVCVGGLLGNCLVMYVILRHTKMKTATNIYIFNLALADTLVLLTLPFQGTDILLGFWPFGNALCKTVIAIDYYNMFTSTFTLTAMSVDRYVAICHPIRALDVRTSSKAQAVNVAIWALASVVGVPVAIMGSAQVEDEEIECLVEIPTPQDYWGPVFAICIFLFSFIVPVLVISVCYSLMIRRLRGVRLLSGSREKDRNLRRITRLVLVVVAVFVGCWTPVQVFVLAQGLGVQPSSETAVAILRFCTALGYVNSCLNPILYAFLDENFKACFRKFCCASALRRDVQVSDRVRSIAKDVALACKTSETVPRPA |
| UniProt | P41146 |
| Protein Data Bank | 5dhh, 5dhg, 4ea3 |
| GPCR-HGmod model | P41146 |
| 3D structure model | This structure is from PDB ID 5dhh. |
| BioLiP | BL0328039,BL0328040, BL0328041,BL0328042, BL0227293,BL0227294 |
| Therapeutic Target Database | T52921 |
| ChEMBL | CHEMBL2014 |
| IUPHAR | 320 |
| DrugBank | BE0002378 |
| Name | BDBM21842 |
|---|---|
| Molecular formula | C79H129N27O22 |
| IUPAC name | (2S)-5-amino-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S,3R)-2-[[(2S)-2-[[2-[[2-[[(2S)-2-amino-3-phenylpropanoyl]amino]acetyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]acetyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]-3-hydroxypropanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]-4-methylpentanoyl]amino]propanoyl]amino]-4-oxobutanoyl]amino]-5-oxopentanoic acid |
| Molecular weight | 1809.07 |
| Hydrogen bond acceptor | 27 |
| Hydrogen bond donor | 28 |
| XlogP | -11.0 |
| Synonyms | OFQ 1-17 |
| Inchi Key | PULGYDLMFSFVBL-SMFNREODSA-N |
| Inchi ID | InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 |
| PubChem CID | 44361543 |
| ChEMBL | CHEMBL396460 |
| IUPHAR | 1681 |
| BindingDB | 50004178, 21842 |
| DrugBank | N/A |
Structure | 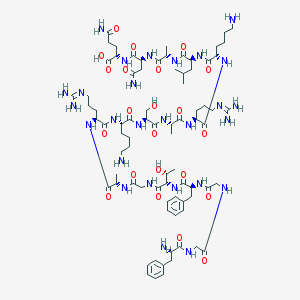 |
| Lipinski's druglikeness | This ligand has more than 5 hydrogen bond donor. This ligand has more than 10 hydrogen bond acceptor. This ligand is heavier than 500 daltons. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 10.0 % | PMID25284251 | ChEMBL |
| Activity | 38.0 % | PMID25284251 | ChEMBL |
| Activity | 62.0 % | PMID25284251 | ChEMBL |
| Activity | 100.0 % | PMID21866885 | ChEMBL |
| Activity | 733.0 % | PMID12431062 | ChEMBL |
| Bmax | 723.0 fmol/mg | PMID19879767 | ChEMBL |
| Bmax | 1712.0 fmol | PMID25284251 | ChEMBL |
| EC50 | 0.08 nM | PMID12372523 | ChEMBL |
| EC50 | 0.08 nM | PMID12372523 | BindingDB |
| EC50 | 0.09 nM | PMID15380196 | ChEMBL |
| EC50 | 0.09 nM | PMID15380196 | BindingDB |
| EC50 | 0.1148 nM | PMID15743186 | ChEMBL |
| EC50 | 0.158489 - 3.98108 nM | PMID9353393 | IUPHAR |
| EC50 | 0.2089 nM | MedChemComm, (2014) 5:7:973 | ChEMBL |
| EC50 | 0.23 nM | PMID27035422 | BindingDB |
| EC50 | 0.23 nM | PMID27035422 | ChEMBL |
| EC50 | 0.389 nM | PMID19473027 | ChEMBL |
| EC50 | 0.389045 nM | PMID19473027 | BindingDB |
| EC50 | 0.45 nM | PMID12431054 | BindingDB,ChEMBL |
| EC50 | 0.912 nM | PMID17490886 | ChEMBL |
| EC50 | 1.047 nM | PMID15743186 | ChEMBL |
| EC50 | 1.1 nM | PMID19879767 | ChEMBL |
| EC50 | 1.5 nM | PMID19125610, PMID20875743 | BindingDB,ChEMBL |
| EC50 | 3.6 nM | PMID24657054 | BindingDB,ChEMBL |
| EC50 | 3.91 nM | PMID18068993 | ChEMBL |
| EC50 | 4.0 - | PMID14684324 | ChEMBL |
| EC50 | 4.0 nM | PMID14684324 | BindingDB |
| EC50 | 8.1 nM | PMID21866885 | BindingDB,ChEMBL |
| EC50 | 13.0 nM | PMID19879767 | ChEMBL |
| EC50 | 24.0 nM | PMID12431062 | BindingDB,ChEMBL |
| EC50 | 63.0 nM | PMID10753470 | BindingDB,ChEMBL |
| Efficacy | 100.0 % | PMID24657054 | ChEMBL |
| Emax | 10.98 - | PMID15743186 | ChEMBL |
| Emax | 98.0 % | PMID15380196 | ChEMBL |
| Emax | 100.0 % | PMID19125610, PMID20875743, PMID18068993, PMID12431054, PMID17490886 | ChEMBL |
| Emax | 103.0 % | PMID15743186 | ChEMBL |
| Emax | 231.0 % | PMID19473027 | ChEMBL |
| IC50 | 0.084 nM | PMID25284251 | ChEMBL |
| IC50 | 0.084 nM | PMID25284251 | BindingDB |
| IC50 | 0.21 nM | PMID19879767 | ChEMBL |
| IC50 | 0.29 nM | PMID23582449 | ChEMBL |
| IC50 | 0.6 nM | PMID18068993 | ChEMBL |
| IC50 | 0.65 nM | PMID25284251 | BindingDB |
| IC50 | 0.65 nM | PMID25284251 | ChEMBL |
| IC50 | 0.72 nM | PMID19879767 | ChEMBL |
| IC50 | 1.3 nM | PMID26988801 | BindingDB,ChEMBL |
| IC50 | 1.5 nM | PMID23466604 | ChEMBL |
| IC50 | 1.7 nM | PMID18983139 | ChEMBL |
| IC50 | 1.8 nM | PMID25284251 | BindingDB |
| IC50 | 1.83 nM | PMID25284251 | ChEMBL |
| IC50 | 2.1 nM | PMID18588282 | ChEMBL |
| Intrinsic activity | 1.0 - | MedChemComm, (2014) 5:7:973 | ChEMBL |
| Kb | 2.59 nM | PMID24678969 | ChEMBL |
| Kd | 0.092 nM | PMID19879767 | ChEMBL |
| Kd | 0.19 nM | PMID25284251 | ChEMBL |
| Kd | 0.19 nM | PMID25284251 | BindingDB |
| Ki | 0.0398108 - 0.199526 nM | PMID10369464, PMID12070757, PMID9413015 | IUPHAR |
| Ki | 0.04898 nM | PMID15743186 | ChEMBL |
| Ki | 0.06 nM | PMID14684324 | BindingDB |
| Ki | 0.06 nM | PMID14684324 | ChEMBL |
| Ki | 0.08 nM | PMID21866885 | BindingDB |
| Ki | 0.08 nM | PMID21866885 | ChEMBL |
| Ki | 0.1 nM | PMID12431062 | BindingDB |
| Ki | 0.1 nM | PMID12431062 | ChEMBL |
| Ki | 0.12 nM | PMID24657054 | ChEMBL |
| Ki | 0.12 nM | PMID24657054 | BindingDB |
| Ki | 0.13 nM | PMID18232652 | BindingDB,ChEMBL |
| Ki | 0.18 nM | PMID15380196 | ChEMBL |
| Ki | 0.18 nM | PMID15380196 | BindingDB |
| Ki | 0.214 nM | PMID24678969 | BindingDB |
| Ki | 0.214 nM | PMID24678969 | ChEMBL |
| Ki | 0.3 nM | PMID12431054 | BindingDB |
| Ki | 0.3 nM | PMID12431054 | ChEMBL |
| Ki | 0.3162 nM | PMID17490886 | ChEMBL |
| Ki | 0.39 nM | PMID19125610, PMID20875743 | BindingDB |
| Ki | 0.39 nM | PMID19125610, PMID20875743 | ChEMBL |
| Ki | 0.48 nM | PMID23466604 | ChEMBL |
| Ki | 0.55 nM | PMID18983139 | ChEMBL |
| Ratio IC50 | 20.0 - | PMID25284251 | ChEMBL |
| Stimulation | 100.0 % | PMID14684324 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417