

You can:
| Name | 5-hydroxytryptamine receptor 1D |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HTR1D |
| Synonym | Serotonin 1D alpha receptor serotonin receptor 1D HTRL Htr1db 5-HT-1D [ Show all ] |
| Disease | Acute migraine Epilepsy Migraine headaches Mood disorder Migraine [ Show all ] |
| Length | 377 |
| Amino acid sequence | MSPLNQSAEGLPQEASNRSLNATETSEAWDPRTLQALKISLAVVLSVITLATVLSNAFVLTTILLTRKLHTPANYLIGSLATTDLLVSILVMPISIAYTITHTWNFGQILCDIWLSSDITCCTASILHLCVIALDRYWAITDALEYSKRRTAGHAATMIAIVWAISICISIPPLFWRQAKAQEEMSDCLVNTSQISYTIYSTCGAFYIPSVLLIILYGRIYRAARNRILNPPSLYGKRFTTAHLITGSAGSSLCSLNSSLHEGHSHSAGSPLFFNHVKIKLADSALERKRISAARERKATKILGIILGAFIICWLPFFVVSLVLPICRDSCWIHPALFDFFTWLGYLNSLINPIIYTVFNEEFRQAFQKIVPFRKAS |
| UniProt | P28221 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P28221 |
| 3D structure model | This predicted structure model is from GPCR-EXP P28221. |
| BioLiP | N/A |
| Therapeutic Target Database | T11072 |
| ChEMBL | CHEMBL1983 |
| IUPHAR | 3 |
| DrugBank | BE0000659 |
| Name | sumatriptan |
|---|---|
| Molecular formula | C14H21N3O2S |
| IUPAC name | 1-[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]-N-methylmethanesulfonamide |
| Molecular weight | 295.401 |
| Hydrogen bond acceptor | 4 |
| Hydrogen bond donor | 2 |
| XlogP | 0.9 |
| Synonyms | AVP825 C14H21N3O2S GR 43175X Imigran Recovery L000584 [ Show all ] |
| Inchi Key | KQKPFRSPSRPDEB-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3 |
| PubChem CID | 5358 |
| ChEMBL | CHEMBL128 |
| IUPHAR | 54 |
| BindingDB | 50005835 |
| DrugBank | DB00669 |
Structure | 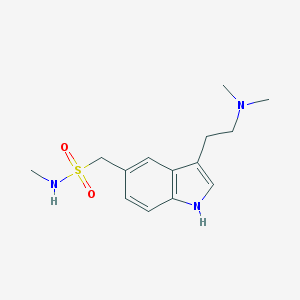 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| EC50 | 4.3 nM | PMID9986723 | BindingDB,ChEMBL |
| EC50 | 5.3 nM | PMID14643336 | BindingDB,ChEMBL |
| EC50 | 12.0 nM | PMID10585208 | BindingDB,ChEMBL |
| EC50 | 14.0 nM | PMID10052975, PMID10052976 | BindingDB,ChEMBL |
| EC50 | 16.0 nM | PMID9357515, PMID9357514 | BindingDB,ChEMBL |
| EC50 | 30.0 nM | , Bioorg. Med. Chem. Lett., (1995) 5:7:663 | BindingDB,ChEMBL |
| EC50 | 220.0 nM | PMID9632357 | BindingDB,ChEMBL |
| ED50 | 317.0 nM | PMID8071931 | ChEMBL |
| Efficacy | 100.0 % | PMID9357515, PMID10585208 | ChEMBL |
| Emax | 93.0 % | PMID9986723 | ChEMBL |
| IC50 | 2.951 nM | PMID11262079 | ChEMBL |
| IC50 | 5.0 nM | PMID9357515, PMID9357514 | BindingDB,ChEMBL |
| IC50 | 6.7 nM | PMID10052975, PMID10052976 | BindingDB,ChEMBL |
| IC50 | 6.8 nM | PMID10585208 | BindingDB,ChEMBL |
| IC50 | 39.81 nM | PMID7752204 | BindingDB,ChEMBL |
| IC50 | 100.0 nM | , Bioorg. Med. Chem. Lett., (1995) 5:22:2649 | BindingDB,ChEMBL |
| IC50 | 2400.0 nM | PMID9357515 | BindingDB,ChEMBL |
| Intrinsic activity | 0.83 - | PMID7658443 | ChEMBL |
| Ki | 1.2 nM | PMID18507369 | BindingDB,ChEMBL |
| Ki | 1.2 nM | PMID18507369 | PDSP |
| Ki | 1.7 nM | PMID8935801 | PDSP,BindingDB |
| Ki | 1.99 nM | PMID10611634 | PDSP,BindingDB |
| Ki | 1.99 - 10.0 nM | PMID10611634, PMID10193663, PMID1565658, PMID8967979, PMID1652050 | IUPHAR |
| Ki | 2.0 nM | PMID9632349 | BindingDB,ChEMBL |
| Ki | 3.38 nM | PMID7984267 | PDSP,BindingDB |
| Ki | 3.4 nM | PMID14741277, PMID14505640 | BindingDB,ChEMBL |
| Ki | 3.7 nM | PMID1652050 | PDSP,BindingDB |
| Ki | 3.71 nM | PMID7984267 | PDSP,BindingDB |
| Ki | 4.4 nM | PMID9986723 | BindingDB,ChEMBL |
| Ki | 5.5 nM | PMID10937729 | BindingDB,ChEMBL |
| Ki | 5.7 nM | PMID8941384 | BindingDB,ChEMBL |
| Ki | 7.7 nM | PMID1565658 | PDSP,BindingDB |
| Ki | 8.4 nM | PMID9303569 | PDSP,BindingDB |
| Ki | 8.5 nM | , PMID8960551, Bioorg. Med. Chem. Lett., (1995) 5:7:663, PMID7658447 | BindingDB,ChEMBL |
| Ki | 8.6 nM | PMID9871581 | BindingDB,ChEMBL |
| Ki | 11.0 nM | PMID10853656 | BindingDB,ChEMBL |
| Ki | 23.0 nM | N/A | BindingDB |
| Ki | 23.1 nM | Bioorg. Med. Chem. Lett., (1995) 5:7:663 | ChEMBL |
| Ki | 30.0 nM | PMID8941384 | BindingDB,ChEMBL |
| p[A50] | 6.6 - | PMID7658443 | ChEMBL |
| Ratio | 3.8 - | PMID8941384 | ChEMBL |
| Relative efficacy | 100.0 % | PMID10052976 | ChEMBL |
| Selectivity ratio | 16.0 - | PMID9357514 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417