

You can:
| Name | Metabotropic glutamate receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | GRM1 |
| Synonym | SCAR13 MGLUR1 mGlu1 receptor metabotropic glutamate receptor 1 GPRC1A [ Show all ] |
| Disease | Alzheimer disease; Huntington's disease Neurological disease Neuropathic pain Schizophrenia Pain |
| Length | 1194 |
| Amino acid sequence | MVGLLLFFFPAIFLEVSLLPRSPGRKVLLAGASSQRSVARMDGDVIIGALFSVHHQPPAEKVPERKCGEIREQYGIQRVEAMFHTLDKINADPVLLPNITLGSEIRDSCWHSSVALEQSIEFIRDSLISIRDEKDGINRCLPDGQSLPPGRTKKPIAGVIGPGSSSVAIQVQNLLQLFDIPQIAYSATSIDLSDKTLYKYFLRVVPSDTLQARAMLDIVKRYNWTYVSAVHTEGNYGESGMDAFKELAAQEGLCIAHSDKIYSNAGEKSFDRLLRKLRERLPKARVVVCFCEGMTVRGLLSAMRRLGVVGEFSLIGSDGWADRDEVIEGYEVEANGGITIKLQSPEVRSFDDYFLKLRLDTNTRNPWFPEFWQHRFQCRLPGHLLENPNFKRICTGNESLEENYVQDSKMGFVINAIYAMAHGLQNMHHALCPGHVGLCDAMKPIDGSKLLDFLIKSSFIGVSGEEVWFDEKGDAPGRYDIMNLQYTEANRYDYVHVGTWHEGVLNIDDYKIQMNKSGVVRSVCSEPCLKGQIKVIRKGEVSCCWICTACKENEYVQDEFTCKACDLGWWPNADLTGCEPIPVRYLEWSNIESIIAIAFSCLGILVTLFVTLIFVLYRDTPVVKSSSRELCYIILAGIFLGYVCPFTLIAKPTTTSCYLQRLLVGLSSAMCYSALVTKTNRIARILAGSKKKICTRKPRFMSAWAQVIIASILISVQLTLVVTLIIMEPPMPILSYPSIKEVYLICNTSNLGVVAPLGYNGLLIMSCTYYAFKTRNVPANFNEAKYIAFTMYTTCIIWLAFVPIYFGSNYKIITTCFAVSLSVTVALGCMFTPKMYIIIAKPERNVRSAFTTSDVVRMHVGDGKLPCRSNTFLNIFRRKKAGAGNANSNGKSVSWSEPGGGQVPKGQHMWHRLSVHVKTNETACNQTAVIKPLTKSYQGSGKSLTFSDTSTKTLYNVEEEEDAQPIRFSPPGSPSMVVHRRVPSAATTPPLPSHLTAEETPLFLAEPALPKGLPPPLQQQQQPPPQQKSLMDQLQGVVSNFSTAIPDFHAVLAGPGGPGNGLRSLYPPPPPPQHLQMLPLQLSTFGEELVSPPADDDDDSERFKLLQEYVYEHEREGNTEEDELEEEEEDLQAASKLTPDDSPALTPPSPFRDSVASGSSVPSSPVSESVLCTPPNVSYASVILRDYKQSSSTL |
| UniProt | Q13255 |
| Protein Data Bank | 4or2, 3ks9 |
| GPCR-HGmod model | N/A |
| 3D structure model | This structure is from PDB ID 4or2. |
| BioLiP | BL0271871,BL0271876, BL0271872,BL0271873,BL0271874,, BL0174211,BL0174213, BL0174210,BL0174212 |
| Therapeutic Target Database | T27137 |
| ChEMBL | CHEMBL3772 |
| IUPHAR | 289 |
| DrugBank | BE0000824 |
| Name | CHEMBL1269131 |
|---|---|
| Molecular formula | C31H48N6O3 |
| IUPAC name | N-[(2S)-1-[3-[4-(3-aminopropylamino)butylamino]propylamino]-1-oxo-6-[(2-phenylacetyl)amino]hexan-2-yl]benzamide |
| Molecular weight | 552.764 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 6 |
| XlogP | 2.2 |
| Synonyms | (S)-N-(22-amino-2,9-dioxo-1-phenyl-3,10,14,19-tetraazadocosan-8-yl)benzamide BDBM50329482 |
| Inchi Key | AWQSDFYHCFZOJA-NDEPHWFRSA-N |
| Inchi ID | InChI=1S/C31H48N6O3/c32-18-11-21-33-19-9-10-20-34-22-12-24-36-31(40)28(37-30(39)27-15-5-2-6-16-27)17-7-8-23-35-29(38)25-26-13-3-1-4-14-26/h1-6,13-16,28,33-34H,7-12,17-25,32H2,(H,35,38)(H,36,40)(H,37,39)/t28-/m0/s1 |
| PubChem CID | 52946879 |
| ChEMBL | CHEMBL1269131 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | N/A |
Structure | 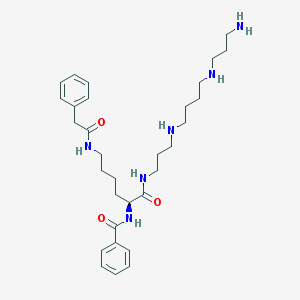 |
| Lipinski's druglikeness | This ligand has more than 5 hydrogen bond donor. This ligand is heavier than 500 daltons. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Inhibition | 35.0 % | PMID20873775 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417