

You can:
| Name | Alpha-1A adrenergic receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADRA1A |
| Synonym | alpha1a ADRA1C ADRA1L1 adrenergic alpha 1c receptor adrenergic receptor alpha 1c [ Show all ] |
| Disease | Urinary incontinence Benign prostatic hyperplasia Cognitive disorders Female sexual dysfunction Glaucoma [ Show all ] |
| Length | 466 |
| Amino acid sequence | MVFLSGNASDSSNCTQPPAPVNISKAILLGVILGGLILFGVLGNILVILSVACHRHLHSVTHYYIVNLAVADLLLTSTVLPFSAIFEVLGYWAFGRVFCNIWAAVDVLCCTASIMGLCIISIDRYIGVSYPLRYPTIVTQRRGLMALLCVWALSLVISIGPLFGWRQPAPEDETICQINEEPGYVLFSALGSFYLPLAIILVMYCRVYVVAKRESRGLKSGLKTDKSDSEQVTLRIHRKNAPAGGSGMASAKTKTHFSVRLLKFSREKKAAKTLGIVVGCFVLCWLPFFLVMPIGSFFPDFKPSETVFKIVFWLGYLNSCINPIIYPCSSQEFKKAFQNVLRIQCLCRKQSSKHALGYTLHPPSQAVEGQHKDMVRIPVGSRETFYRISKTDGVCEWKFFSSMPRGSARITVSKDQSSCTTARVRSKSFLQVCCCVGPSTPSLDKNHQVPTIKVHTISLSENGEEV |
| UniProt | P35348 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | P35348 |
| 3D structure model | This predicted structure model is from GPCR-EXP P35348. |
| BioLiP | N/A |
| Therapeutic Target Database | T92609 |
| ChEMBL | CHEMBL229 |
| IUPHAR | 22 |
| DrugBank | BE0000501 |
| Name | prazosin |
|---|---|
| Molecular formula | C19H21N5O4 |
| IUPAC name | [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone |
| Molecular weight | 383.408 |
| Hydrogen bond acceptor | 8 |
| Hydrogen bond donor | 1 |
| XlogP | 2.0 |
| Synonyms | HSDB 3298 1-(4-amino-6,7-dimethoxy-2-quinazolinyl-4-(2-furanylcarbonyl)) piperrazine KBio1_000375 2-[4-(Furan-2-Ylcarbonyl)piperazin-1-Yl]-6,7-Dimethoxyquinazolin-4-Amine KBio3_000732 [ Show all ] |
| Inchi Key | IENZQIKPVFGBNW-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) |
| PubChem CID | 4893 |
| ChEMBL | CHEMBL2 |
| IUPHAR | 503 |
| BindingDB | 29568 |
| DrugBank | DB00457 |
Structure | 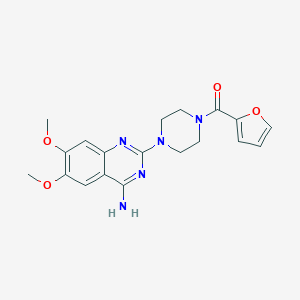 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Ke | 1.1 nM | PMID21900013, PMID24630561 | ChEMBL |
| Ke(app) | 1.1 nM | PMID26475518 | ChEMBL |
| Ki | 0.03 nM | PMID7815325 | BindingDB |
| Ki | 0.04 nM | PMID25557493, PMID24805037 | BindingDB |
| Ki | 0.04 nM | PMID25557493, PMID24805037 | ChEMBL |
| Ki | 0.06 nM | PMID10611634 | PDSP,BindingDB |
| Ki | 0.09 nM | PMID6149136 | BindingDB |
| Ki | 0.11 nM | PMID7855217 | BindingDB |
| Ki | 0.12 nM | PMID7815325 | BindingDB |
| Ki | 0.125893 - 1.0 nM | PMID9249248, PMID7651358, PMID9490024, PMID10369480, PMID10334511 | IUPHAR |
| Ki | 0.1995 nM | PMID9135028 | ChEMBL |
| Ki | 0.2 nM | PMID16420037 | BindingDB |
| Ki | 0.21 nM | PMID8396931 | BindingDB |
| Ki | 0.27 nM | PMID7752182 | BindingDB,ChEMBL |
| Ki | 0.28 nM | PMID11132243 | BindingDB |
| Ki | 0.31 nM | PMID7815325 | BindingDB |
| Ki | 0.32 nM | PMID16420037 | BindingDB |
| Ki | 0.39 nM | PMID9548811 | BindingDB,ChEMBL |
| Ki | 0.51 nM | PMID7658428 | BindingDB,ChEMBL |
| Ki | 0.58 nM | PMID10522703 | BindingDB |
| Ki | 0.58 nM | PMID9857099, PMID9888842, PMID10522703 | BindingDB,ChEMBL |
| Ki | 0.588 nM | Med Chem Res, (2011) 20:9:1455 | ChEMBL |
| Ki | 0.5888 nM | PMID15633998, PMID14584940, Med Chem Res, (2011) 20:9:1455, PMID9822553 | ChEMBL |
| Ki | 0.59 nM | PMID24365159 | ChEMBL |
| Ki | 0.6 nM | PMID10579841 | BindingDB,ChEMBL |
| Ki | 0.61 nM | PMID11448222 | BindingDB,ChEMBL |
| Ki | 0.69 nM | PMID12065700 | BindingDB |
| Ki | 0.691831 nM | PMID12065700 | PDSP |
| Ki | 1.862 nM | PMID24365159 | ChEMBL |
| Selectivity | 0.0057 - | PMID6133953 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417