

You can:
| Name | Glucagon-like peptide 1 receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | GLP1R |
| Synonym | glucagon-like peptide 1 receptor GLP-1R GLP-1-R GLP-1 receptor |
| Disease | Type 1/2 diabetes Type 1 diabetes Obesity Non-insulin dependent diabetes Non-alcoholic steatohepatitis [ Show all ] |
| Length | 463 |
| Amino acid sequence | MAGAPGPLRLALLLLGMVGRAGPRPQGATVSLWETVQKWREYRRQCQRSLTEDPPPATDLFCNRTFDEYACWPDGEPGSFVNVSCPWYLPWASSVPQGHVYRFCTAEGLWLQKDNSSLPWRDLSECEESKRGERSSPEEQLLFLYIIYTVGYALSFSALVIASAILLGFRHLHCTRNYIHLNLFASFILRALSVFIKDAALKWMYSTAAQQHQWDGLLSYQDSLSCRLVFLLMQYCVAANYYWLLVEGVYLYTLLAFSVLSEQWIFRLYVSIGWGVPLLFVVPWGIVKYLYEDEGCWTRNSNMNYWLIIRLPILFAIGVNFLIFVRVICIVVSKLKANLMCKTDIKCRLAKSTLTLIPLLGTHEVIFAFVMDEHARGTLRFIKLFTELSFTSFQGLMVAILYCFVNNEVQLEFRKSWERWRLEHLHIQRDSSMKPLKCPTSSLSSGATAGSSMYTATCQASCS |
| UniProt | P43220 |
| Protein Data Bank | 5vex, 3c59, 3c5t, 5nx2, 3iol, 4zgm, 5otu, 5vew, 5otw, 5otx, 5otv |
| GPCR-HGmod model | P43220 |
| 3D structure model | This structure is from PDB ID 5vex. |
| BioLiP | BL0418498,BL0418499, BL0143794, BL0143795, BL0167479, BL0167480, BL0324354, BL0324355,BL0324356, BL0378791,BL0378792, BL0379513,BL0379514, BL0418500,BL0418501, BL0380967, BL0418494,BL0418495, BL0418496,BL0418497, BL0143732, BL0143731, BL0380966 |
| Therapeutic Target Database | T36075 |
| ChEMBL | CHEMBL1784 |
| IUPHAR | 249 |
| DrugBank | BE0000857 |
| Name | MLS000051886 |
|---|---|
| Molecular formula | C19H16ClN3O3 |
| IUPAC name | 2-[(4-chlorophenyl)carbamoylamino]-N-(furan-2-ylmethyl)benzamide |
| Molecular weight | 369.805 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 3 |
| XlogP | 3.5 |
| Synonyms | 2-[[(4-chloroanilino)-oxomethyl]amino]-N-(2-furanylmethyl)benzamide CBKinase1_011139 MolPort-001-908-286 AC1LMK22 CHEMBL1508615 [ Show all ] |
| Inchi Key | ARCJTCXIQFJWDK-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C19H16ClN3O3/c20-13-7-9-14(10-8-13)22-19(25)23-17-6-2-1-5-16(17)18(24)21-12-15-4-3-11-26-15/h1-11H,12H2,(H,21,24)(H2,22,23,25) |
| PubChem CID | 1131627 |
| ChEMBL | CHEMBL1508615 |
| IUPHAR | N/A |
| BindingDB | 32790 |
| DrugBank | N/A |
Structure | 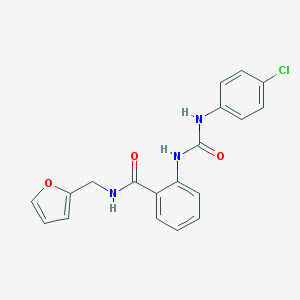 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Potency | 15848.9 nM | PubChem BioAssay data set | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417