

You can:
| Name | 5-hydroxytryptamine receptor 2C |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HTR2C |
| Synonym | Serotonin receptor 2C serotonin 1c receptor 5-HT1C 5-HT2C 5-HT-2C [ Show all ] |
| Disease | Pain Sleep initiation and maintenance disorders; Primary insomnia; Schizophrenia Unspecified Depression Drug abuse [ Show all ] |
| Length | 458 |
| Amino acid sequence | MVNLRNAVHSFLVHLIGLLVWQCDISVSPVAAIVTDIFNTSDGGRFKFPDGVQNWPALSIVIIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVWPLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWAISIGVSVPIPVIGLRDEEKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYCLTIYVLRRQALMLLHGHTEEPPGLSLDFLKCCKRNTAEEENSANPNQDQNARRRKKKERRPRGTMQAINNERKASKVLGIVFFVFLIMWCPFFITNILSVLCEKSCNQKLMEKLLNVFVWIGYVCSGINPLVYTLFNKIYRRAFSNYLRCNYKVEKKPPVRQIPRVAATALSGRELNVNIYRHTNEPVIEKASDNEPGIEMQVENLELPVNPSSVVSERISSV |
| UniProt | P28335 |
| Protein Data Bank | 6bqg, 6bqh |
| GPCR-HGmod model | P28335 |
| 3D structure model | This structure is from PDB ID 6bqg. |
| BioLiP | BL0404805, BL0404806 |
| Therapeutic Target Database | T83813 |
| ChEMBL | CHEMBL225 |
| IUPHAR | 8 |
| DrugBank | BE0004957, BE0004881, BE0000533 |
| Name | Ketanserin |
|---|---|
| Molecular formula | C22H22FN3O3 |
| IUPAC name | 3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-1H-quinazoline-2,4-dione |
| Molecular weight | 395.434 |
| Hydrogen bond acceptor | 5 |
| Hydrogen bond donor | 1 |
| XlogP | 2.6 |
| Synonyms | MolPort-003-666-642 A838000 NCGC00021146-08 AOB5639 NSC-758959 [ Show all ] |
| Inchi Key | FPCCSQOGAWCVBH-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) |
| PubChem CID | 3822 |
| ChEMBL | CHEMBL51 |
| IUPHAR | 88, 197 |
| BindingDB | 21395 |
| DrugBank | DB12465 |
Structure | 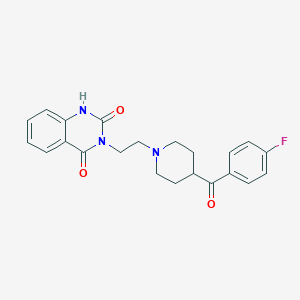 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Ki | 2.5 nM | PMID7855217 | BindingDB |
| Ki | 16.98 nM | PMID11882920 | BindingDB |
| Ki | 16.9824 nM | PMID11882920 | PDSP |
| Ki | 31.6228 - 158.489 nM | PMID15322733, PMID8845011, PMID12738034, PMID10217294 | IUPHAR |
| Ki | 43.65 nM | PMID11754579 | ChEMBL |
| Ki | 47.86 nM | PMID9225287 | PDSP,BindingDB |
| Ki | 50.11 nM | PMID7582481 | PDSP,BindingDB |
| Ki | 61.6595 nM | PMID15322733 | PDSP |
| Ki | 61.66 nM | PMID15322733 | BindingDB |
| Ki | 100.0 nM | PMID3543362 | BindingDB,ChEMBL |
| Ki | 161.0 nM | PMID8632342, PMID8845011 | PDSP,BindingDB |
| Ki | 186.0 nM | Hoyer et al., PMID1986 | PDSP |
| Ki | 199.52 nM | PMID7582481 | PDSP,BindingDB |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417