

You can:
| Name | 5-hydroxytryptamine receptor 2A |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | HTR2A |
| Synonym | 5-HT-2 serotonin receptor 2A serotonin 5HT-2 receptor 5-HT-2A 5-HT2A receptor [ Show all ] |
| Disease | Depression Unspecified Diabetes Erythropoietic porphyria Fibromyalgia [ Show all ] |
| Length | 471 |
| Amino acid sequence | MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGCLSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKSSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV |
| UniProt | P28223 |
| Protein Data Bank | 6a93, 6a94 |
| GPCR-HGmod model | P28223 |
| 3D structure model | This structure is from PDB ID 6a93. |
| BioLiP | BL0441025,BL0441028, BL0441031, BL0441030,BL0441033, BL0441029,BL0441032, BL0441026, BL0441024,BL0441027 |
| Therapeutic Target Database | T32060 |
| ChEMBL | CHEMBL224 |
| IUPHAR | 6 |
| DrugBank | BE0000451 |
| Name | trifluoperazine |
|---|---|
| Molecular formula | C21H24F3N3S |
| IUPAC name | 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine |
| Molecular weight | 407.499 |
| Hydrogen bond acceptor | 7 |
| Hydrogen bond donor | 0 |
| XlogP | 5.0 |
| Synonyms | NCGC00013226-04 CCG-37306 NCGC00013226-12 D08636 Eskazine (TN) [ Show all ] |
| Inchi Key | ZEWQUBUPAILYHI-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 |
| PubChem CID | 5566 |
| ChEMBL | CHEMBL422 |
| IUPHAR | 214 |
| BindingDB | 79181 |
| DrugBank | DB00831 |
Structure | 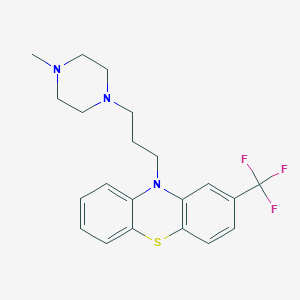 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 99.0 % | PMID17407813 | ChEMBL |
| Ki | 7.4 nM | http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W84-42KD8D9-7&_coverDate=01%2F31%2F2001&_alid=93617739&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=6644&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5e5748d1ff8f5317fb6669f7ddc989f6 | PDSP |
| Ki | 8.8 nM | http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W84-42KD8D9-7&_coverDate=01%2F31%2F2001&_alid=93617739&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=6644&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5e5748d1ff8f5317fb6669f7ddc989f6 | PDSP |
| Ki | 12.0 nM | http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W84-42KD8D9-7&_coverDate=01%2F31%2F2001&_alid=93617739&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=6644&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5e5748d1ff8f5317fb6669f7ddc989f6 | PDSP |
| Ki | 12.5893 nM | PMID12629531 | IUPHAR |
| Ki | 13.0 nM | PMID12629531 | PDSP,BindingDB |
| Ki | 14.0 nM | Wander et al., PMID1987 | PDSP |
| Ki | 18.24 nM | Andorn et al., PMID1984 | PDSP |
| Ki | 135.0 nM | PMID9015795 | PDSP,BindingDB |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417