

You can:
| Name | D(1A) dopamine receptor |
|---|---|
| Species | Rattus norvegicus (Rat) |
| Gene | Drd1 |
| Synonym | D1 receptor D1A DADR dopamine D1 receptor Dopamine-1A receptor [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 446 |
| Amino acid sequence | MAPNTSTMDEAGLPAERDFSFRILTACFLSLLILSTLLGNTLVCAAVIRFRHLRSKVTNFFVISLAVSDLLVAVLVMPWKAVAEIAGFWPLGPFCNIWVAFDIMCSTASILNLCVISVDRYWAISSPFQYERKMTPKAAFILISVAWTLSVLISFIPVQLSWHKAKPTWPLDGNFTSLEDTEDDNCDTRLSRTYAISSSLISFYIPVAIMIVTYTSIYRIAQKQIRRISALERAAVHAKNCQTTAGNGNPVECAQSESSFKMSFKRETKVLKTLSVIMGVFVCCWLPFFISNCMVPFCGSEETQPFCIDSITFDVFVWFGWANSSLNPIIYAFNADFQKAFSTLLGCYRLCPTTNNAIETVSINNNGAVVFSSHHEPRGSISKDCNLVYLIPHAVGSSEDLKKEEAGGIAKPLEKLSPALSVILDYDTDVSLEKIQPVTHSGQHST |
| UniProt | P18901 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL265 |
| IUPHAR | 214 |
| DrugBank | N/A |
| Name | UNII-UGT5535REQ |
|---|---|
| Molecular formula | C17H18ClNO |
| IUPAC name | (5R)-8-chloro-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol |
| Molecular weight | 287.787 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 1 |
| XlogP | 4.0 |
| Synonyms | GOTMKOSCLKVOGG-OAHLLOKOSA-N PDSP2_000612 SCH23390 2,3,4,5-Tetrahydro-8-chloro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol (R)- BRD-K45435259-001-01-6 [ Show all ] |
| Inchi Key | GOTMKOSCLKVOGG-OAHLLOKOSA-N |
| Inchi ID | InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 |
| PubChem CID | 3036864 |
| ChEMBL | CHEMBL62 |
| IUPHAR | N/A |
| BindingDB | 82247 |
| DrugBank | N/A |
Structure | 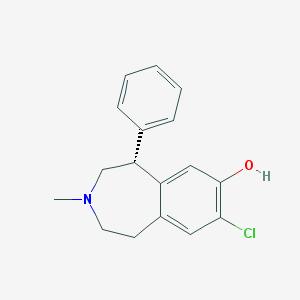 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| -Log K0.5 | 9.4 - | PMID10464009 | ChEMBL |
| Change | -35.0 % | PMID12723940 | ChEMBL |
| Change | 52.0 % | PMID12723940 | ChEMBL |
| Change | 62.0 % | PMID12723940 | ChEMBL |
| IC50 | 0.57 nM | PMID7830274 | BindingDB,ChEMBL |
| IC50 | 1.0 nM | PMID1971308 | BindingDB,ChEMBL |
| IC50 | 1.01 nM | PMID7914538 | BindingDB,ChEMBL |
| IC50 | 1.3 nM | PMID2405158 | BindingDB,ChEMBL |
| IC50 | 4.3 nM | PMID9804688 | BindingDB,ChEMBL |
| K 0.5 | 0.4 nM | PMID10072690 | ChEMBL |
| K0.5 | 0.3 nM | PMID8558526 | ChEMBL |
| K0.5 | 0.5 nM | PMID8568818, PMID9216832 | ChEMBL |
| K0.5 | 0.52 nM | PMID7636869 | ChEMBL |
| Ki | 0.12 nM | PMID10843230 | ChEMBL |
| Ki | 0.12 nM | PMID10843230 | BindingDB |
| Ki | 0.14 nM | PMID2415793, PMID1397049, PMID3039136, PMID7855175 | BindingDB,ChEMBL |
| Ki | 0.2 nM | PMID8584042 | BindingDB |
| Ki | 0.21 nM | PMID7473556 | BindingDB,ChEMBL |
| Ki | 0.3 nM | PMID6387355, Bioorg. Med. Chem. Lett., (1992) 2:5:399 | BindingDB,ChEMBL |
| Ki | 0.3 nM | N/A | BindingDB |
| Ki | 0.34 nM | PMID12023552 | BindingDB |
| Ki | 0.37 nM | PMID10327430 | BindingDB |
| Ki | 0.39 nM | PMID2525621 | BindingDB,ChEMBL |
| Ki | 0.4 nM | PMID2666667, PMID2905002, PMID7996543 | BindingDB,ChEMBL |
| Ki | 0.4 nM | PMID7996543 | BindingDB |
| Ki | 0.4266 nM | PMID7996543 | ChEMBL |
| Ki | 0.43 nM | PMID3050089 | ChEMBL |
| Ki | 0.43 nM | PMID3050089 | BindingDB |
| Ki | 0.73 nM | PMID8863801 | BindingDB,ChEMBL |
| Ki | 0.7413 nM | PMID10691686 | ChEMBL |
| Ki | 0.8 nM | PMID7902811 | BindingDB |
| Ki | 0.9 nM | PMID1833546 | ChEMBL |
| Ki | 0.9 nM | PMID1833546 | BindingDB |
| Ki | 1.4 nM | PMID10227113 | BindingDB |
| Ki | 17.0 nM | PMID8558526 | BindingDB |
| Ki | 173.0 nM | PMID1397049 | BindingDB |
| Ki | 178.0 nM | PMID2531826 | BindingDB |
| Ki | 676.0 nM | PMID7996543 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417