

You can:
| Name | Prostaglandin E2 receptor EP1 subtype |
|---|---|
| Species | Mus musculus (Mouse) |
| Gene | Ptger1 |
| Synonym | EP1 prostanoid receptor EP1 receptor PGE receptor EP1 subtype PGE2 receptor EP1 subtype prostaglandin E receptor 1 (subtype EP1), 42kDa [ Show all ] |
| Disease | N/A for non-human GPCRs |
| Length | 405 |
| Amino acid sequence | MSPCGLNLSLADEAATCATPRLPNTSVVLPTGDNGTSPALPIFSMTLGAVSNVLALALLAQVAGRMRRRRSAATFLLFVASLLAIDLAGHVIPGALVLRLYTAGRAPAGGACHFLGGCMVFFGLCPLLLGCGMAVERCVGVTQPLIHAARVSVARARLALAVLAAMALAVALLPLVHVGRYELQYPGTWCFISLGPRGGWRQALLAGLFAGLGLAALLAALVCNTLSGLALLRARWRRRRSRRFRKTAGPDDRRRWGSRGPRLASASSASSITSATATLRSSRGGGSARRVHAHDVEMVGQLVGIMVVSCICWSPLLVLVVLAIGGWNSNSLQRPLFLAVRLASWNQILDPWVYILLRQAMLRQLLRLLPLRVSAKGGPTELGLTKSAWEASSLRSSRHSGFSHL |
| UniProt | P35375 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL2181 |
| IUPHAR | 340 |
| DrugBank | N/A |
| Name | CHEMBL2315047 |
|---|---|
| Molecular formula | C24H21Cl2FN2O2 |
| IUPAC name | [6-[[5-chloro-2-[(4-chloro-2-fluorophenyl)methoxy]phenyl]methyl]pyridin-2-yl]-pyrrolidin-1-ylmethanone |
| Molecular weight | 459.342 |
| Hydrogen bond acceptor | 4 |
| Hydrogen bond donor | 0 |
| XlogP | 5.9 |
| Synonyms | BDBM50424392 |
| Inchi Key | ZDUFQAJHKGKMQW-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C24H21Cl2FN2O2/c25-18-8-9-23(31-15-16-6-7-19(26)14-21(16)27)17(12-18)13-20-4-3-5-22(28-20)24(30)29-10-1-2-11-29/h3-9,12,14H,1-2,10-11,13,15H2 |
| PubChem CID | 71519328 |
| ChEMBL | CHEMBL2315047 |
| IUPHAR | N/A |
| BindingDB | 50424392 |
| DrugBank | N/A |
Structure | 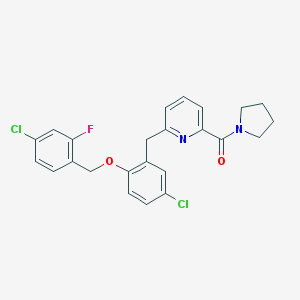 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Kd | 15800.0 nM | PMID23218714 | BindingDB,ChEMBL |
| Kd | 15848.9 nM | PMID23218714 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417