

You can:
| Name | Adenosine receptor A3 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | ADORA3 |
| Synonym | ARA3 Adenosine receptor A3 A3AR A3 receptor TGPCR1 |
| Disease | Cerebrovascular ischaemia Malaria Ischemia Inflammation Hepatocellular carcinoma; Hepatitis C virus infection [ Show all ] |
| Length | 318 |
| Amino acid sequence | MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIAVGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKRVTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYFSFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFALSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKACVVCHPSDSLDTSIEKNSE |
| UniProt | P0DMS8 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | T36059 |
| ChEMBL | CHEMBL256 |
| IUPHAR | 21 |
| DrugBank | BE0000354 |
| Name | N6-Cyclopentyladenosine |
|---|---|
| Molecular formula | C15H21N5O4 |
| IUPAC name | (2R,3R,4S,5R)-2-[6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol |
| Molecular weight | 335.364 |
| Hydrogen bond acceptor | 8 |
| Hydrogen bond donor | 4 |
| XlogP | 0.9 |
| Synonyms | NCGC00023909-06 UNII-7LG47VG1ID (2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol AC-27402 cid_657378 [ Show all ] |
| Inchi Key | SQMWSBKSHWARHU-SDBHATRESA-N |
| Inchi ID | InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 |
| PubChem CID | 657378 |
| ChEMBL | CHEMBL68738 |
| IUPHAR | 380 |
| BindingDB | 25400 |
| DrugBank | N/A |
Structure | 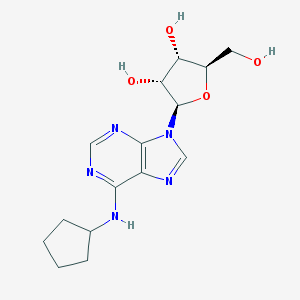 |
| Lipinski's druglikeness | This ligand satisfies Lipinski's rule of five. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Activity | 72.0 % | PMID16366590 | ChEMBL |
| EC50 | 242.0 nM | PMID12238926 | BindingDB,ChEMBL |
| EC50 | 7980.0 nM | PMID10841798 | BindingDB,ChEMBL |
| Efficacy | 97.0 % | PMID12238926 | ChEMBL |
| Ki | 39.8107 - 100.0 nM | PMID8234299, PMID15476669, PMID9459566, PMID16518376 | IUPHAR |
| Ki | 43.0 nM | PMID9459566 | BindingDB |
| Ki | 72.0 nM | PMID23200243, PMID26390077, PMID12238926 | BindingDB,ChEMBL |
| Ki | 89.12 nM | PMID8234299 | BindingDB |
| Ki | 120.0 nM | PMID10212125, PMID10999489 | BindingDB,ChEMBL |
| Ki | 137.0 nM | PMID12238926 | BindingDB,ChEMBL |
| Ki | 142.0 nM | PMID12238926 | BindingDB,ChEMBL |
| Ki | 240.0 nM | PMID16366590 | BindingDB,ChEMBL |
| Ki | 280.0 nM | PMID18637670 | BindingDB,ChEMBL |
| Ki | 281.0 nM | PMID18258439, PMID15771447, PMID11520205 | BindingDB,ChEMBL |
| Ki | 615.0 nM | PMID12238926 | BindingDB,ChEMBL |
| Ki | 1490.0 nM | PMID23200243 | ChEMBL |
| Ki | 3250.0 nM | PMID20188574 | BindingDB,ChEMBL |
| Ki | 18600.0 nM | PMID17933541 | BindingDB,ChEMBL |
| Max | 100.0 % | PMID10841798 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417