

You can:
| Name | Type-1 angiotensin II receptor |
|---|---|
| Species | Oryctolagus cuniculus (Rabbit) |
| Gene | AGTR1 |
| Synonym | Angiotensin II type-1 receptor AT1 |
| Disease | N/A for non-human GPCRs |
| Length | 359 |
| Amino acid sequence | MMLNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLAVIVIYFYMKLKTVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNYLCKIASASVSFNLYASVFLLTCLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLLAGLASLPAIIHRNVFFIENTNITVCAFHYESQNSTLPIGLGLTKNILGFLFPFLIILTSYTLIWKALKKAYEIQKNKPRNDDIFKIIMAIVLFFFFSWVPHQIFTFLDVLIQLGVIHDCRIADIVDTAMPITICIAYFNNCLNPLFYGFLGKKFKKYFLQLLKYIPPKAKSHSNLSTKMSTLSYRPSDNVSSSSKKPVPCFEVE |
| UniProt | P34976 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | N/A |
| ChEMBL | CHEMBL3948 |
| IUPHAR | N/A |
| DrugBank | N/A |
| Name | CHEMBL299151 |
|---|---|
| Molecular formula | C28H33N5O3 |
| IUPAC name | ethyl (2E)-2-[4-butyl-4-methyl-6-oxo-1-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]piperidin-2-ylidene]acetate |
| Molecular weight | 487.604 |
| Hydrogen bond acceptor | 6 |
| Hydrogen bond donor | 1 |
| XlogP | 5.2 |
| Synonyms | BDBM50280319 [4-Butyl-4-methyl-6-oxo-1-[2''-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-piperidin-(2E)-ylidene]-acetic acid ethyl ester |
| Inchi Key | AOQKRBLMQYAZFA-CJLVFECKSA-N |
| Inchi ID | InChI=1S/C28H33N5O3/c1-4-6-15-28(3)17-22(16-26(35)36-5-2)33(25(34)18-28)19-20-11-13-21(14-12-20)23-9-7-8-10-24(23)27-29-31-32-30-27/h7-14,16H,4-6,15,17-19H2,1-3H3,(H,29,30,31,32)/b22-16+ |
| PubChem CID | 44295090 |
| ChEMBL | CHEMBL299151 |
| IUPHAR | N/A |
| BindingDB | 50280319 |
| DrugBank | N/A |
Structure | 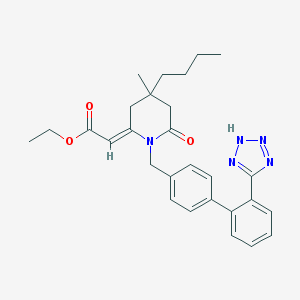 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Kd | 31.62 nM | , Bioorg. Med. Chem. Lett., (1994) 4:1:87 | BindingDB,ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417