

You can:
| Name | Type-1 angiotensin II receptor |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | AGTR1 |
| Synonym | Type-1 angiotensin II receptor HAT1R Agtr-1a type-1A angiotensin II receptor AT1 [ Show all ] |
| Disease | Metabolic syndrome x Myocardial infarction Hypertension Restenosis Alzheimer disease [ Show all ] |
| Length | 359 |
| Amino acid sequence | MILNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLKTVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNYLCKIASASVSFNLYASVFLLTCLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLLAGLASLPAIIHRNVFFIENTNITVCAFHYESQNSTLPIGLGLTKNILGFLFPFLIILTSYTLIWKALKKAYEIQKNKPRNDDIFKIIMAIVLFFFFSWIPHQIFTFLDVLIQLGIIRDCRIADIVDTAMPITICIAYFNNCLNPLFYGFLGKKFKRYFLQLLKYIPPKAKSHSNLSTKMSTLSYRPSDNVSSSTKKPAPCFEVE |
| UniProt | P30556 |
| Protein Data Bank | 6do1, 4zud, 4yay |
| GPCR-HGmod model | P30556 |
| 3D structure model | This structure is from PDB ID 6do1. |
| BioLiP | BL0312790, BL0326733, BL0439004,BL0439005 |
| Therapeutic Target Database | T74456 |
| ChEMBL | CHEMBL227 |
| IUPHAR | 34 |
| DrugBank | BE0000062 |
| Name | CHEBI:58506 |
|---|---|
| Molecular formula | C50H71N13O12 |
| IUPAC name | (3S)-3-azaniumyl-4-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[(2S)-2-[[(1S)-1-carboxylato-2-phenylethyl]carbamoyl]pyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-(diaminomethylideneazaniumyl)-1-oxopentan-2-yl]amino]-4-oxobutanoate |
| Molecular weight | 1046.2 |
| Hydrogen bond acceptor | 13 |
| Hydrogen bond donor | 12 |
| XlogP | -0.4 |
| Synonyms | Ile(5)-angiotensin II dizwitterion |
| Inchi Key | CZGUSIXMZVURDU-JZXHSEFVSA-N |
| Inchi ID | InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 |
| PubChem CID | 45266664 |
| ChEMBL | CHEMBL408403 |
| IUPHAR | 2504 |
| BindingDB | 50236697 |
| DrugBank | DB11842 |
Structure | 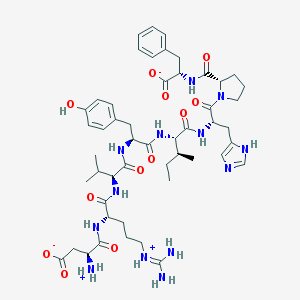 |
| Lipinski's druglikeness | This ligand has more than 5 hydrogen bond donor. This ligand has more than 10 hydrogen bond acceptor. This ligand is heavier than 500 daltons. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Activity | 0.84 - | PMID18077343 | ChEMBL |
| EC50 | 0.489779 nM | PMID26824643 | BindingDB |
| EC50 | 0.4898 nM | PMID26824643 | ChEMBL |
| EC50 | 0.52 nM | PMID26824643 | BindingDB |
| EC50 | 0.52 nM | PMID26824643 | ChEMBL |
| IC50 | 0.501187 - 1.0 nM | PMID10193788 | IUPHAR |
| IC50 | 0.7244 nM | PMID26824643 | ChEMBL |
| IC50 | 0.724436 nM | PMID26824643 | BindingDB |
| Kd | 2.0 nM | PMID8709104 | BindingDB,ChEMBL |
| Ki | 0.16 nM | PMID26824643 | BindingDB |
| Ki | 0.16 nM | PMID26824643 | ChEMBL |
| Ki | 0.24 nM | PMID26824643 | ChEMBL |
| Ki | 0.24 nM | PMID26824643 | BindingDB |
| Ki | 0.9 nM | PMID20146480 | ChEMBL |
| Relative affinity | 100.0 - | PMID8709104 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417